
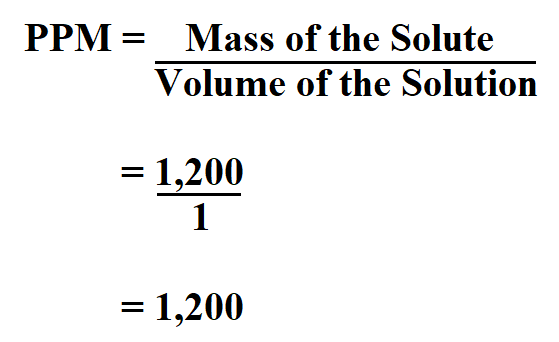
12.5 mls of 100 ppm in 50 ml volume will give a 25 ppm solution = no of mls for req volĮxample : Make up 50 mls vol of 25 ppm from 100 ppm standard.Ģ5 x 50 / 100 = 12.5 mls. The Formula below can be used to calculate the the V1 component only. This means that 66.7 mls of the 6.00ppm solution diluted to a final volume of 4 liters will give a concentration of 0.100 ppm. This equation applies to all dilution problems.Ĭ1 (initial conc) x V1 (initial volume) = C2 (final conc) x V2 (final volume)Įxample : What volume of 6.00 ppm solution must be used to give 4.00 liters of a 0.100 ppm solution? Hence, weigh out 1.432g KH 2PO 4 and dissolve in 1 liter volume to make a 1000 ppm PO 4 standard.Ĭlick this link for Atomic absorption standards Make a 1000 ppm phosphate standard using the salt KH 2PO 4ġg PO 4 in relation to FW of salt = 136.09 / 95 = 1.432g. Hence, weigh out 2.542g NaCl and dissolve in 1 liter volume to make a 1000 ppm Na standard.Į.g. Make a 1000 ppm standard of Na using the salt NaCl.ġg Na in relation to FW of salt = 58.44 / 23 = 2.542g. nitric or hydrochloric acid, and make up to the mark in 1 liter volume deionised water.Į.g. From the pure metal : weigh out accurately 1.000g of metal, dissolve in 1 : 1 conc. PPB = Parts per billion = ug/L = ng/g = ng/ml = pg/mg = 10 -9ġ. Ppm = ug/g =ug/ml = ng/mg = pg/ug = 10 -6ġ gram pure element disolved in 1000ml = 1000 ppm PPM is derived from the fact that the density of water is taken as 1kg/L = 1,000,000 mg/L, and 1mg/L is 1mg/1,000,000mg or one part in one million. One thousanth of a gram is one milligram and 1000 ml is one liter, so that 1 ppm = 1 mg per liter = mg/Liter. One gram in 1000 ml is 1000 ppm and one thousandth of a gram (0.001g) in 1000 ml is one ppm. PPM is a term used in chemistry to denote a very, very low concentration of a solution.
#How to calculate ppm serial#
Worked Examples: Converting Percentage Concentration to ppmįollow these 4 steps to convert percentage concentration to parts.PPM conversion values and serial dilutions :

M/v (w/v) and m/m (w/w) concentrations and parts per million will be discussed in the following section (after the worked examples of percentage concentration and ppm below).

V/v concentration is NOT the same as a %(v/v) concentration M/m concentration is NOT the same as a %(m/m) concentration W/v concentration is NOT the same as a %(w/v) concentration M/v concentration is NOT the same as a %(m/v) concentration

Where x is the value of the percentage concentration This is true for other similar percentage concentrations:įor mass/volume percentage concentrations (m/v% or w/v%):įor mass/mass percentage concentrations (m/m% or w/w%): We could write a mathematical expression equating parts per hundred (a percentage) and parts per million (ppm) as shown below: How would we convert that to a concentration in parts per million (ppm) ? This means that there are 9 000 parts of NaCl in every 1 000 000 parts of the solution.īut the concentration of a solution is sometimes given as a percentage.Ī percentage concentration tells you how many parts of solute are present in 100 parts of solution.įor example, the ethanol content in wine is often given as about 12%(v/v), that is, 12% of the volume of the wine is ethanol, or, there are 12 parts of ethanol in every 100 parts of solution. Recall that, in general, concentration tells you how much solute is present in a solution.Ī concentration in parts per million (ppm) tells you how many parts of solute are present in 1 000 000 parts of solution.įor example, a saline solution is a dilute aqueous solution of sodium chloride, NaCl (aq), with a concentration of 9 000 ppm. Parts per million and Percentage Concentration Calculations


 0 kommentar(er)
0 kommentar(er)
